in a separatory funnel which is the organic layer
4.4: Which Level is Which?
- Page ID
- 93532
Density
Information technology is essential that you know whether the aqueous layer is to a higher place or beneath the healthful layer in the separatory funnel, as it dictates which layer is kept and which is in time discarded. 2 immiscible solvents will whole sle atop unity another supported differences in tightness. The solvent with the lower density will rest happening high, and the denser solution will build on the bottom.
To the highest degree not-halogenated constitutional solvents experience densities less than 1 g/cubic centimetre, so leave drift atop an liquid solution (if they are unmixable). A famed exception is that halogenated solvents are denser than water (have densities greater than 1 g/mL), and so will instead pass below sedimentary solutions (Table 4.1 and Figure 4.8).
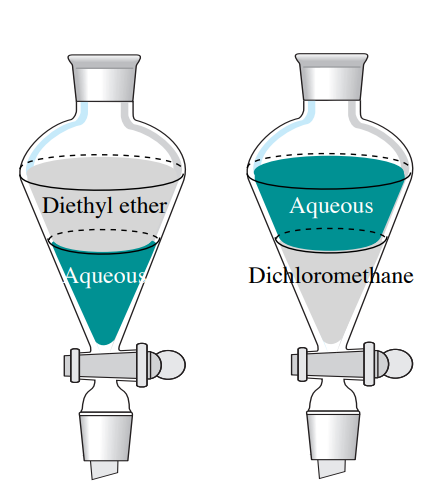
| Solvent | Density (g/mL) |
|---|---|
| Pentane | 0.626 |
| Fossil oi Ether (mixture of C5 - C6 hydrocarbons) | 0.653 |
| Hexanes (mixture of 6 hydrocarbons) | 0.655 |
| Ethyl ether | 0.713 |
| Ethyl radical ethanoate | 0.902 |
| Water | 0.998 |
| Dicholoromethane (CH2Cl2) | 1.33 |
| Chloroform (CHCl3) | 1.49 |
Many solutions used in separatory funnels are fairly dilute, so the density of the solution is approximately the same as the tightness of the solvent. For example, if mixing diethyl ether and a \(10\% \: \ce{NaOH} \left( aq \right)\) solution in a separatory funnel, knowledge of the exact concentration of the \(10\% \: \ce{NaOH}\) solution is not necessary. A \(10\% \: \ce{NaOH} \left( aq \right)\) solution is \(90\%\) water (by mass), signification the tightness should comprise fairly close to the density of water (approximately \(1 \: \text{g/mL}\)). The true tightness of a \(10\% \: \cerium{NaOH} \left( aq \right wing)\) resolution is \(1.1089 \: \text edition{g/mL}\), a value only slenderly greater than the density of water. The ether will be the top layer in that situation.
At that place are times, however, when so may solute particles are dissolved that a solution's density is much greater than the solvent compactness. For deterrent example, a saturated \(\ce{NaCl} \remaining( aq \right)\) solution has a density around \(1.2 \: \text{g/millilitre}\) (importantly greater than the density of water), and can cause breakup problems with solvents of replaceable densities like dichloromethane.
How to Limit the Aqueous Layer
Result densities Crataegus laevigata be used to predict which layer is organic and which is sedimentary in a separatory funnel, but there are other methods that can be useful in this decision. If unsure which stratum is aqueous and which layer is organic, do one of the favourable things:
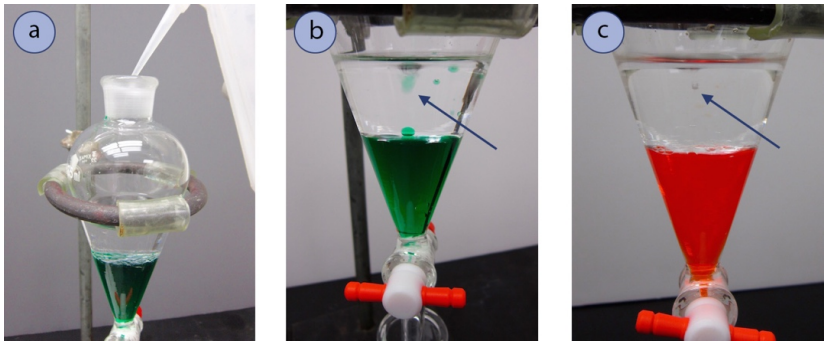
- Add a bit of pee from a pip-squeak bottle to the separatory funnel (Visualize 4.9a) and watch where the urine droplets go.
If the top layer is binary compound, the water droplets should mix with the top layer, and they will look as if they disappear. If the bottom layer is aqueous, the pee droplets will fall through the top layer to mix with the merchantman layer (as indicated by an pointer in Figure 4.9b+c). If it is difficult to course where the water droplets pop off, also keep track of the mass of the layers: whichever layer increases with the addition of water is the aqueous layer.
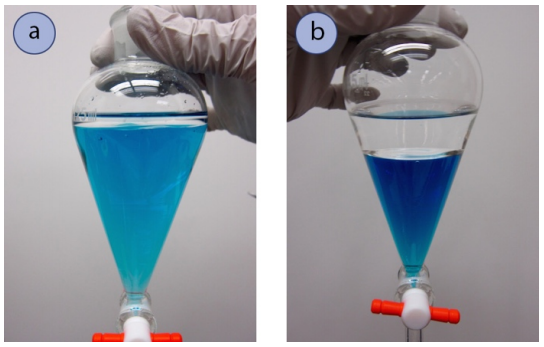
- Consider congenator volumes of aqueous and organic solvents, based along quantities used in the experiment.
Figure 4.10a shows a \(125 \: \text{mL}\) separatory funnel shape containing \(10 \: \text{mL}\) hexane and \(100 \: \text{mL}\) water (tinted with blue dye). If these were the quantities misused in an experiment, the binary compound layer would have to be the lower layer as it is and so much large. Although univocal in this case, information technology is important to know that the odd shape of the separatory funnel may cause you to misjudge volumes. A separatory funnel shape with equal volumes of aqueous and organic layers is shown in Build 4.10b, although the layers rise to different heights in the funnel.
\(^1\)The solvents listed in Postpone 4.1 are immaculate compounds except for crude oil ether and hexanes. "Crude oil Ethoxyethane" contains pentane, 2-methylbutane, 2,2-dimethylpropane, n-hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and 2,3-dimethylbutane. "Hexanes" contains 2-methylpentane, 3-methylpentane, n-hexane, and methylcyclopentane.
Contributor
-
Lisa Nichols (Butte Community College).Organic Chemistry Science laborator Techniques is licensed under a Creative Commonality Attribution-NonCommercial-NoDerivatives 4.0 International License. Complete text is purchasable online.
in a separatory funnel which is the organic layer
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_Lab_Techniques_(Nichols)/04%3A_Extraction/4.04%3A_Which_Layer_is_Which
Posting Komentar untuk "in a separatory funnel which is the organic layer"